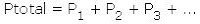Dalton’s law states that in a mixture of different gases, such as air, the sum of the partial pressures of all the gases equals the total pressure. The partial pressure of a gas is the pressure that gas would exert if it was the only gas present. This law, expressed mathematically, is:
where each subscripted P value is the partial pressure of a different gas.
Air, for example, consists mostly of nitrogen and oxygen, with small quantities of argon, water vapor, and carbon dioxide. The partial pressure of nitrogen accounts for 78 percent of the total pressure exerted by Earth’s atmosphere; oxygen accounts for 21 percent; and argon accounts for 0.9 percent. Together, these three gases account for 99.9 percent of air pressure. Water vapor, carbon dioxide, and all the other trace gases present in the atmosphere combined contribute only a tenth of a percent.
Air, for example, consists mostly of nitrogen and oxygen, with small quantities of argon, water vapor, and carbon dioxide. The partial pressure of nitrogen accounts for 78 percent of the total pressure exerted by Earth’s atmosphere; oxygen accounts for 21 percent; and argon accounts for 0.9 percent. Together, these three gases account for 99.9 percent of air pressure. Water vapor, carbon dioxide, and all the other trace gases present in the atmosphere combined contribute only a tenth of a percent.