.
 If two charged objects in contact have the same capacitance, they divide the charge evenly. Suppose, for example, that one object has a charge of +4 coulombs and the other a charge of +8 coulombs. When they touch, charge will flow from the 8-coulomb object to the 4-coulomb object until each has a charge of +6 coulombs. If each object originally had a charge of +6 coulombs, no charge would flow between them.
If two charged objects in contact have the same capacitance, they divide the charge evenly. Suppose, for example, that one object has a charge of +4 coulombs and the other a charge of +8 coulombs. When they touch, charge will flow from the 8-coulomb object to the 4-coulomb object until each has a charge of +6 coulombs. If each object originally had a charge of +6 coulombs, no charge would flow between them.
If two objects have different capacitances, they divide the charge in proportion to their capacitances. If an object with a capacitance of 10 farads touches an object with a capacitance of 5 farads, the 10-farad object will end up with twice the amount of charge of the 5-farad object. Suppose that the objects are oppositely charged and that one has a charge of +20 coulombs and the other a charge of -8 coulombs. Their total charge is therefore +12 coulombs. After they touch, the 10-farad object will have a charge of +8 coulombs and the 5-farad object will have +4 coulombs.
Objects with opposite charges attract each other, and objects with similar charges repel each other. Coulomb’s law, formulated by French physicist Charles Augustin de Coulomb during the late 18th century, quantifies the strength of the attraction or repulsion. This law states that the force between two charged objects is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The greater the charges on the objects, the larger the force between them; the greater the distance between the objects, the lesser the force between them. The unit of electric charge, also named after Coulomb, is equal to the combined charges of 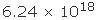 protons (or electrons).
protons (or electrons).
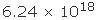 protons (or electrons).
protons (or electrons).  If two charged objects in contact have the same capacitance, they divide the charge evenly. Suppose, for example, that one object has a charge of +4 coulombs and the other a charge of +8 coulombs. When they touch, charge will flow from the 8-coulomb object to the 4-coulomb object until each has a charge of +6 coulombs. If each object originally had a charge of +6 coulombs, no charge would flow between them.
If two charged objects in contact have the same capacitance, they divide the charge evenly. Suppose, for example, that one object has a charge of +4 coulombs and the other a charge of +8 coulombs. When they touch, charge will flow from the 8-coulomb object to the 4-coulomb object until each has a charge of +6 coulombs. If each object originally had a charge of +6 coulombs, no charge would flow between them.If two objects have different capacitances, they divide the charge in proportion to their capacitances. If an object with a capacitance of 10 farads touches an object with a capacitance of 5 farads, the 10-farad object will end up with twice the amount of charge of the 5-farad object. Suppose that the objects are oppositely charged and that one has a charge of +20 coulombs and the other a charge of -8 coulombs. Their total charge is therefore +12 coulombs. After they touch, the 10-farad object will have a charge of +8 coulombs and the 5-farad object will have +4 coulombs.



