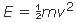
E = (ma)d
where a is the acceleration applied to the mass, m, and d is the distance through which a acts. The relationships between kinetic and potential energy and among the concepts of force, distance, acceleration, and energy can be illustrated by the lifting and dropping of an object.
When the object is lifted from a surface a vertical force is applied to the object. As this force acts through a distance, energy is transferred to the object. The energy associated with an object held above a surface is termed potential energy. If the object is dropped, the potential energy is converted to kinetic energy. See Mechanics.



